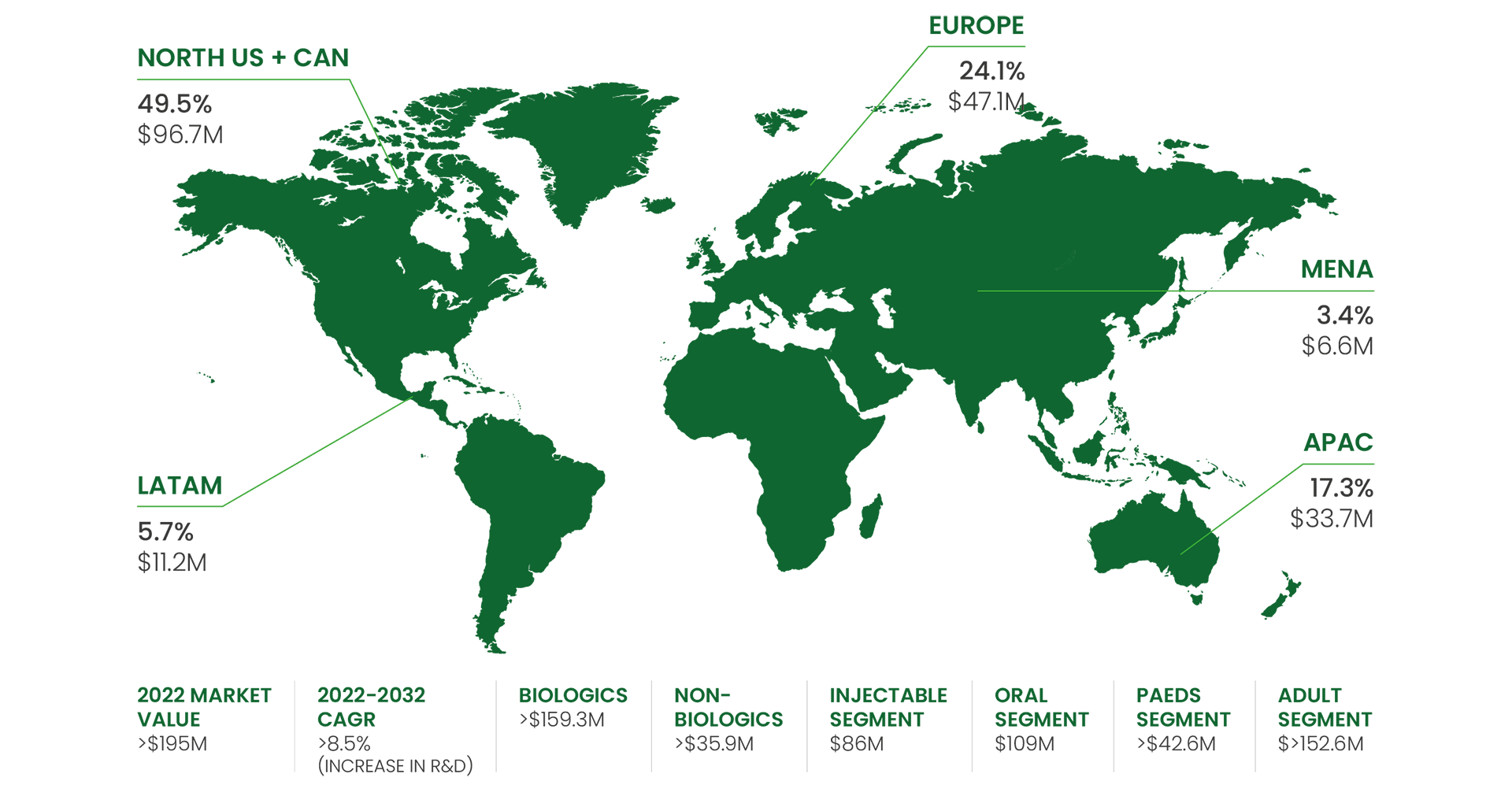
Did you know that over 30 million people in Europe live with a rare disease, often lacking access to effective treatments due to the complexities of market entry? This challenge highlights a missed opportunity. According to 2022 global market insights, the landscape of rare disease treatment development is experiencing a dynamic shift. While specific regional breakdowns may vary by source, the data consistently highlights a flourishing market. The total market size for rare disease treatments surpassed USD 134.91 billion in 2022 and is projected to continue this upward trajectory, with a Compound Annual Growth Rate (CAGR) exceeding 8.5% through 2032. This growth is fuelled by a global increase in research and development efforts, with a significant portion of companies developing these life-saving therapies originating from North America (49.5%), Europe (24.1%), APAC (17.3%), LATAM (5.7%), and MENA 3.4%).
However, a gap exists between potential and realized market access. Many companies, particularly those outside of Europe, choose to pursue FDA approval due to its perceived simplicity. The complexity of the European Medicines Agency (EMA) registration process can be a significant hurdle, potentially delaying access to millions of patients in need. Partner Rare bridges this gap by providing the expertise and guidance to navigate the EMA process efficiently, ensuring your life-saving therapy reaches the European market and maximizes its potential to impact patient lives.
Navigating the journey from clinical trials to market access requires a deep understanding of the European landscape. Partner Rare’s team of experts have extensive experience in designing and conducting clinical trials tailored to meet EU regulatory requirements, while simultaneously conducting market access research to inform successful commercialization strategies. This ensures your therapy not only meets scientific standards but also addresses the specific needs of European patients and healthcare systems.

Our team isn’t just about experience; we are your gateway to success in Europe. We leverage a robust global network and possess an intricate understanding of European regulations. We are comprised of C-suite executives who have led successful rare disease companies, biotech owners with a deep understanding of the entrepreneurial journey, and renowned professors at the forefront of scientific advancements. Additionally, we house experts in HTA submissions and pricing and reimbursement, ensuring your therapy demonstrates its value proposition to healthcare decision-makers, and can navigate the complexities of securing fair compensation for your life-saving therapy.

Partner Rare understands the importance of patient advocacy. We cultivate extensive contacts with global and European rare disease patient organizations. By collaborating with these groups, we gain valuable insights into patient needs and expectations, ultimately ensuring your therapy addresses a critical unmet medical need. Our team doesn’t just connect you with patients; we actively champion your cause. We leverage our experience in policy change to advocate for policies that improve access to rare disease treatments. Additionally, our team includes globally recognized keynote speakers who can raise awareness of your rare disease assets with the very community that requires it the most. This comprehensive approach ensures your therapy not only reaches patients but also resonates with them on a deeper level.
With Partner Rare by your side, you gain access to a wealth of knowledge, experience, and strategic guidance. We’ll help you navigate the European market efficiently, accelerate market access for your therapy, and ultimately bring hope to patients in need.
For promising start-ups requiring a comprehensive approach, we offer the Partner Forward Accelerator Program. Partner Forward recognizes the challenges associated with rare disease asset development and commercialization. This program is designed to help you lower risks and costs by providing the highest level of mentorship, strategic advisory, and consulting from our in-house rare disease experts at a reduced service cost. Through Partner Forward, you’ll gain access to the resources and guidance needed to propel your therapy towards success in Europe, ultimately accelerating time to market and maximizing its impact on patient lives.
Our expertise extends beyond market access. We understand the importance of securing the right financial partnerships to fuel your success. Our team includes experts in finance partnering and licensing, who can help you explore strategic options to unlock the full potential of your asset. Additionally, our business development specialists will work with you to identify and navigate potential licensing or acquisition opportunities once your therapy achieves relevant milestones.
Throughout the journey, our team of rare disease marketing experts ensures clear and compliant communication with all stakeholders. We’ll craft a compelling narrative that resonates with investors, patients, and healthcare professionals, ensuring your journey is communicated in a professional and effective manner. Partner Rare offers a comprehensive solution, empowering you to bring life-saving therapies to European patients and revolutionize the landscape of rare disease treatment.
If you are ready to unlock the vast potential of Europe for your rare disease therapy, contact Partner Rare today to schedule a meeting.
Let’s discuss your specific development and commercialization goals and explore how our expertise can propel your therapy towards success.
Accelerating Your Innovation’s Journey to Market with our bespoke Accelerator Program.

Your gateway to successful rare disorder asset commercialization in the European market.



